Hydrosphere
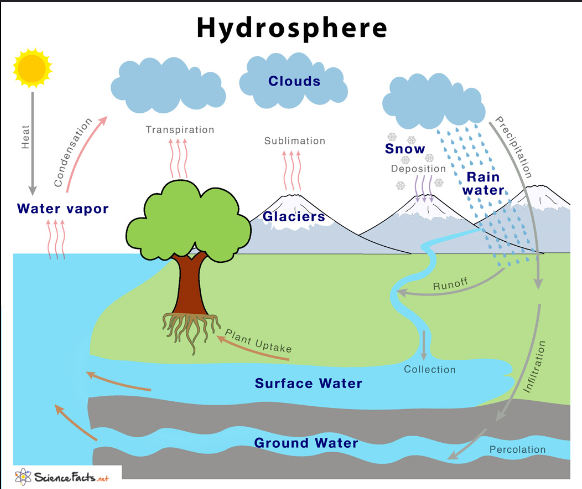
The hydrosphere encompasses all water found on, under, and over the surface of the Earth. It includes:
- Oceans and Seas: Cover about 71% of the Earth’s surface and contain about 97.5% of all Earth’s water.
- Lakes and Rivers: Freshwater bodies that play crucial roles in ecosystems and human use.
- Glaciers and Ice Caps: Hold about 68.7% of the world’s freshwater.
- Groundwater: Water stored underground in aquifers, which accounts for about 30.1% of the world’s freshwater.
- Atmospheric Water: Water vapor and clouds in the atmosphere.
- Soil Moisture: Water held in the soil that is essential for plant growth.
Hydrological Cycle (Water Cycle)
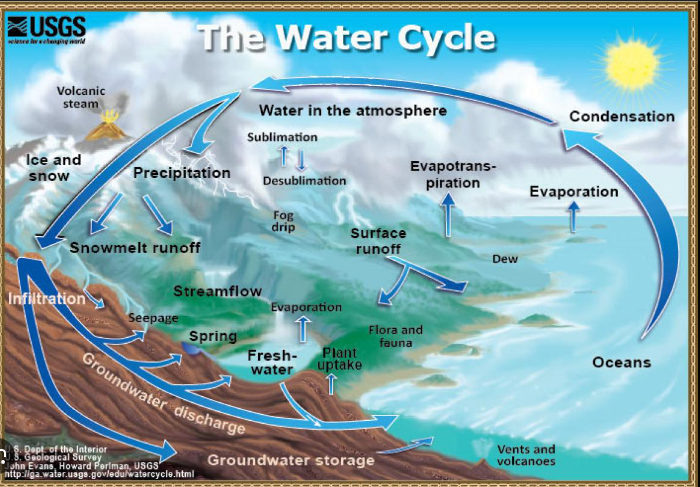
The hydrological cycle describes the continuous movement of water on, above, and below the surface of the Earth. The key processes involved are:
- Evaporation: Water from oceans, lakes, and rivers heats up and turns into water vapor, entering the atmosphere.
- Transpiration: Water absorbed by plants from the soil is released into the atmosphere as water vapor through tiny pores in leaves.
- Condensation: Water vapor in the atmosphere cools and turns into liquid water, forming clouds.
- Precipitation: Water falls from clouds as rain, snow, sleet, or hail, returning to the Earth’s surface.
- Infiltration: Some of the precipitation soaks into the ground, replenishing groundwater.
- Runoff: Water flows over the surface of the land, returning to rivers, lakes, and eventually the oceans.
- Percolation: Water moves downward through soil and rock layers, replenishing aquifers.
The cycle is driven by solar energy and gravity, and it helps in the distribution of heat around the globe, supporting all forms of life.
Natural Water
Natural water refers to water that exists in the environment without significant human alteration. It includes:
- Surface Water:
- Rivers and Streams: Flowing bodies of water that move continuously towards oceans, lakes, or other rivers.
- Lakes and Ponds: Inland bodies of standing water that vary in size.
- Wetlands: Areas where water covers the soil or is present at or near the surface of the soil for varying periods.
- Groundwater:
- Aquifers: Underground layers of water-bearing rock or materials (gravel, sand, silt) from which groundwater can be extracted.
- Wells and Springs: Natural discharge points of groundwater at the Earth’s surface.
- Marine Water:
- Oceans and Seas: Large bodies of saltwater that cover a major part of the Earth’s surface.
Pollutants of Water
Oxygen Demanding Wastes
- Origin: These pollutants are primarily organic matter such as sewage, agricultural runoff, and industrial waste.
- Effects: They require oxygen for decomposition by bacteria. High levels lead to reduced dissolved oxygen (DO) in water, which can cause the death of fish and other aquatic organisms due to oxygen depletion.
Pathogens
- Origin: Pathogens include bacteria, viruses, protozoa, and parasites that enter water through sewage, agricultural runoff, and untreated wastewater.
- Effects: They cause waterborne diseases such as cholera, dysentery, and hepatitis, posing significant health risks to humans and animals.
Nutrients
- Origin: Nutrients such as nitrogen and phosphorus come from agricultural fertilizers, animal waste, sewage, and industrial effluents.
- Effects: Excessive nutrients lead to eutrophication, which causes the overgrowth of algae. When algae die and decompose, they deplete oxygen in the water, resulting in dead zones where aquatic life cannot survive.
Salts
- Origin: Sources include agricultural runoff, industrial effluents, and natural mineral deposits.
- Effects: High salinity can harm aquatic life, reduce agricultural productivity by affecting soil quality, and make water unsuitable for drinking.
Thermal Pollution
- Origin: Discharge of heated water from industrial processes, power plants, and cooling systems.
- Effects: Increased water temperatures lower dissolved oxygen levels and disrupt aquatic ecosystems by altering the metabolic rates of organisms and making them more susceptible to disease.
Heavy Metals
- Origin: Industrial discharges, mining activities, and improper disposal of electronic waste.
- Examples and Effects:
- Lead: Causes neurological damage and developmental issues in children.
- Mercury: Accumulates in the food chain, leading to mercury poisoning.
- Cadmium: Causes kidney damage and bone fractures.
- Arsenic: Linked to skin lesions, cancer, and cardiovascular diseases.
Pesticides
- Origin: Agricultural runoff from the use of chemicals to control pests.
- Effects: Pesticides can be toxic to aquatic life, disrupt reproductive systems, and accumulate in the food chain, affecting both wildlife and humans.
Volatile Organic Compounds (VOCs)
- Origin: Industrial solvents, fuel spills, and the improper disposal of household chemicals.
- Effects: VOCs can cause cancer, liver damage, and kidney damage. They also contaminate groundwater and can make water unsafe for consumption.
River Pollution
Key Parameters:
-
Dissolved Oxygen (DO):
- Definition: The amount of oxygen dissolved in water, essential for aquatic life.
- Impact of Pollution: Oxygen levels decrease due to the presence of organic pollutants which bacteria break down, consuming oxygen in the process. Low DO can lead to the death of fish and other aquatic organisms.
-
5-Day Biochemical Oxygen Demand (BOD) Test:
- Definition: Measures the amount of oxygen required by bacteria to decompose organic matter in water over five days.
- Purpose: Indicates the level of organic pollution. Higher BOD values suggest higher levels of organic pollutants.
-
Seeded BOD Test:
- Definition: Involves adding a known quantity of bacteria (seed) to ensure there are enough bacteria to consume the organic matter.
- Purpose: Used when water samples have insufficient bacteria to naturally decompose the organic matter.
-
BOD Reaction Rate Constants:
- Definition: Constants used to describe the rate at which BOD decreases over time.
- Purpose: Helps in modeling the decomposition process and predicting the oxygen demand over time.
-
Effect of Oxygen Demanding Wastes on River:
- Deoxygenation: The process of reducing DO levels due to the breakdown of organic matter.
- Reaeration: The natural process by which water absorbs oxygen from the atmosphere. It helps replenish DO levels.
-
Chemical Oxygen Demand (COD):
- Definition: Measures the total quantity of oxygen required to chemically oxidize organic and inorganic substances in water.
- Purpose: Indicates the level of both biodegradable and non-biodegradable pollutants.
-
Oil and Greases:
- Sources: Industrial discharges, urban runoff, and oil spills.
- Effects: Form a film on the water surface, reducing oxygen transfer, and harming aquatic life.
-
pH:
- Definition: A measure of how acidic or alkaline water is.
- Impact of Pollution: Pollutants can alter the pH, making the water too acidic or too alkaline, which can be harmful to aquatic organisms.
Lake Pollution
Key Concepts:
-
Eutrophication:
- Definition: The process by which lakes become nutrient-rich, leading to excessive growth of algae and other plants.
- Sources: Mainly from runoff containing fertilizers, sewage, and detergents.
- Effects: When algae die, their decomposition depletes oxygen, causing dead zones and fish kills. It also results in poor water quality and can produce harmful algal blooms that release toxins.
-
Definition, Source, and Effect:
- Definition: Eutrophication is defined by the nutrient enrichment and resulting ecological changes.
- Source: The primary sources include agricultural runoff (high in nitrogen and phosphorus), wastewater discharges, and stormwater runoff.
- Effect: Leads to oxygen depletion, loss of biodiversity, and can affect drinking water supplies and recreational activities.
Groundwater Pollution
Key Concepts:
-
Aquifers:
- Definition: Underground layers of water-bearing rock or materials from which groundwater can be extracted.
- Types: Confined aquifers (overlain by impermeable rock) and unconfined aquifers (directly recharged by surface water).
-
Hydraulic Gradient:
- Definition: The slope of the water table or potentiometric surface.
- Purpose: Drives the flow of groundwater. A steeper gradient means faster flow.
-
Groundwater Flow:
- Definition: The movement of water through the pore spaces or fractures in rocks and sediments.
- Factors Influencing Flow: Permeability of the geological material, pressure differences, and the hydraulic gradient.
Standard and Control
Waste Water Standards
Waste water standards are regulatory benchmarks set by authorities to control the quality of water discharged from various sources to protect public health and the environment. These standards specify acceptable levels of pollutants in wastewater. Key parameters include:
-
Biochemical Oxygen Demand (BOD):
- Definition: The amount of dissolved oxygen needed by aerobic biological organisms to break down organic material in water.
- Standard: Typically, lower BOD values are required to ensure cleaner effluents.
-
Chemical Oxygen Demand (COD):
- Definition: Measures the total quantity of oxygen required to chemically oxidize both organic and inorganic compounds in water.
- Standard: Limits are set to ensure that effluents do not deplete the oxygen levels in receiving waters.
-
Oil and Grease:
- Definition: Organic substances including hydrocarbons, fats, oils, waxes, and related compounds.
- Standard: Set to prevent surface films that reduce oxygen transfer and harm aquatic life.
-
Grease:
- Similar to Oil and Grease: Specific limits are set to control the discharge of greasy substances that can cause blockages and environmental harm.
Water Treatment System (Focus on the subtopics’ headings and gist)
Water treatment systems are designed to remove contaminants from water to make it safe for drinking, industrial use, or safe discharge into the environment. These systems generally include several stages:
-
Coagulation and Flocculation:
- Coagulation: The process of adding chemicals (coagulants) to water to destabilize and aggregate suspended particles.
- Flocculation: Gentle stirring to form larger aggregates (flocs) from the smaller particles formed during coagulation.
-
Sedimentation and Filtration:
- Sedimentation: The process where gravity helps settle the flocs at the bottom of a sedimentation tank.
- Filtration: Passing water through filters (sand, gravel, or membranes) to remove remaining suspended particles and flocs.
-
Disinfection:
- Definition: The process of killing or inactivating harmful microorganisms in water.
- Methods: Chlorination, ultraviolet (UV) radiation, ozonation, and others.
-
Hardness and Alkalinity:
- Hardness: Caused by dissolved minerals, primarily calcium and magnesium.
- Alkalinity: The water’s capacity to neutralize acids, primarily due to bicarbonates, carbonates, and hydroxides.
- Softening: Processes like ion exchange, lime softening, or reverse osmosis to reduce water hardness.
Waste Water Treatment System
Wastewater treatment involves several stages to remove contaminants before water is released back into the environment:
-
Primary Treatment:
- Definition: Physical process to remove large particles and solids from wastewater.
- Processes:
- Screening: Removes large debris.
- Grit Removal: Removes sand, gravel, and other heavy particles.
- Sedimentation: Settles suspended solids in a primary clarifier.
-
Secondary Treatment:
- Definition: Biological process to remove dissolved and suspended organic matter.
- Processes:
- Trickling Filters: Wastewater is sprayed over a bed of rocks or other materials covered with microbial biofilms that degrade organic matter.
- Rotating Biological Contractors: Discs covered with biofilms rotate, exposing microbes to wastewater and air.
- Activated Sludge: Aerated tanks where bacteria break down organic matter; includes a secondary clarifier to settle out the biomass.
- Sludge Treatment: Further treatment of sludge (solid waste) from primary and secondary treatments.
- Oxidation Ponds: Large, shallow ponds where natural processes (algal photosynthesis and bacterial degradation) treat wastewater.
-
Tertiary Treatment:
- Definition: Advanced treatment to remove remaining contaminants after primary and secondary treatment.
- Processes:
- Filtration: Further removal of residual solids.
- Nutrient Removal: Processes to remove nitrogen and phosphorus.
- Disinfection: Final disinfection to kill any remaining pathogens.
Importance of Standards and Control
- Environmental Protection: Ensures that treated water released into natural bodies of water does not harm ecosystems.
- Public Health: Protects drinking water sources from contamination and reduces the risk of waterborne diseases.
- Regulatory Compliance: Helps industries and municipalities comply with legal requirements, avoiding fines and sanctions.
BOD Test (Biochemical Oxygen Demand)
What is BOD?
- Definition: BOD is the amount of dissolved oxygen needed by aerobic microorganisms to decompose the organic matter in water over a specific period, typically five days (BOD₅) at a controlled temperature (usually 20°C).
- Purpose: It measures the amount of biodegradable organic material in water, indicating the level of pollution.
Procedure:
- Sample Collection: Water samples are collected in airtight bottles to prevent any loss of dissolved oxygen or contamination.
- Initial DO Measurement: The dissolved oxygen (DO) level is measured immediately after collection using a DO meter.
- Incubation: The sample is then incubated in the dark at 20°C for five days to prevent photosynthesis, which could alter DO levels.
- Final DO Measurement: After the incubation period, the DO level is measured again.
- BOD Calculation: The BOD value is calculated by subtracting the final DO from the initial DO. This value represents the amount of oxygen consumed by microorganisms during the incubation period.

Significance:
- Low BOD: Indicates good water quality with low levels of organic pollution.
- High BOD: Suggests high levels of organic pollution, leading to oxygen depletion which can harm aquatic life.
Seeded BOD Test
Why Seeded BOD Test?
In some cases, water samples may not contain enough microorganisms to decompose the organic matter effectively. This could be due to the water source, such as highly treated wastewater or water with low microbial activity. The Seeded BOD test adds a known microbial population (seed) to the sample to ensure proper decomposition of organic matter.
Procedure:
- Preparation of Seed: Microorganisms are cultivated from a source like treated wastewater, which is rich in bacteria capable of degrading organic matter. This culture is aerated and allowed to grow for a few days.
- Sample Preparation: Water samples are prepared as in the standard BOD test.
- Addition of Seed: A small volume of the prepared seed culture is added to the water samples to ensure an adequate microbial population.
- Initial DO Measurement: The DO level of the seeded sample is measured.
- Incubation: The sample is incubated at 20°C for five days in the dark.
- Final DO Measurement: The DO level is measured again after incubation.
- BOD Calculation: The BOD value is calculated similarly by subtracting the final DO from the initial DO.

Corrections for Seed Control:
To account for the oxygen demand of the seed itself, a seed control (a blank sample containing only seed in dilution water) is also incubated and its BOD is measured. This value is subtracted from the BOD of the seeded sample to get the true BOD of the water sample.

Importance:
- Accuracy: The Seeded BOD test provides more accurate BOD values for samples with low or absent microbial populations.
- Consistency: Ensures that the BOD test results are consistent and reliable, regardless of the sample’s initial microbial content.