Atmospheric composition
What is the atmosphere?
The atmosphere is a mixture of gases that surrounds the Earth. It helps make life possible by providing us with air to breathe, shielding us from harmful ultraviolet (UV) radiation coming from the Sun, trapping heat to warm the planet, and preventing extreme temperature differences between day and night.
Composition of the Atmosphere
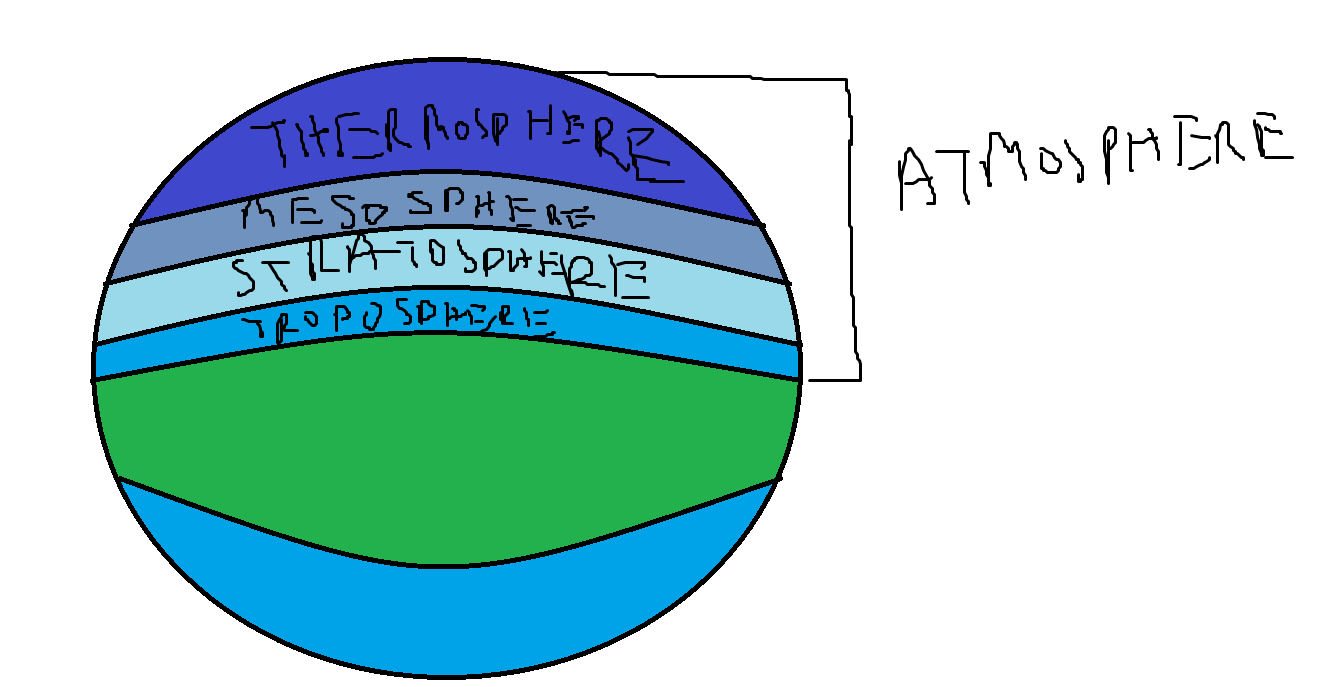
Troposphere
- Definition: The lowest layer of Earth's atmosphere, extending from the surface up to about 8-15 kilometers (5-9 miles) depending on latitude (higher at the equator and lower at the poles).
- Characteristics:
- Contains approximately 75% of the atmosphere's mass and 99% of its water vapor and aerosols.
- Temperature generally decreases with altitude.
- Weather phenomena, such as clouds, rain, and storms, occur in this layer.
- The boundary between the troposphere and stratosphere is called the tropopause.
Stratosphere
- Definition: The layer above the troposphere, extending from the tropopause to about 50 kilometers (31 miles) above Earth’s surface.
- Characteristics:
- Contains the ozone layer, which absorbs and scatters ultraviolet (UV) solar radiation.
- Temperature increases with altitude due to the absorption of UV radiation by ozone.
- The stratosphere is relatively stable and lacks the turbulent mixing found in the troposphere.
- The boundary between the stratosphere and mesosphere is called the stratopause.
Mesosphere
- Definition: The layer above the stratosphere, extending from the stratopause to about 85 kilometers (53 miles) above Earth.
- Characteristics:
- Temperature decreases with altitude, making it the coldest layer of the atmosphere.
- Meteors burn up in this layer due to friction with the atmosphere.
- The mesosphere has very low air pressure and density.
- The boundary between the mesosphere and thermosphere is called the mesopause.
Thermosphere
- Definition: The layer above the mesosphere, extending from the mesopause to about 600 kilometers (373 miles) above Earth.
- Characteristics:
- Temperature increases significantly with altitude, reaching up to 2,500°C (4,500°F) or higher.
- Contains a small fraction of the atmosphere's mass but has very high energy particles.
- The thermosphere is the layer where the auroras (Northern and Southern Lights) occur.
- The International Space Station (ISS) orbits within this layer.
- The boundary above the thermosphere is less defined, gradually transitioning into outer space.
Tropopause
- Definition: The boundary between the troposphere and the stratosphere.
- Characteristics:
- Acts as a cap, limiting the mixing of the troposphere and stratosphere.
- Characterized by a temperature inversion, where temperature stops decreasing with altitude and starts increasing in the stratosphere.
- The height of the tropopause varies with latitude and season.
Mesopause
- Definition: The boundary between the mesosphere and the thermosphere.
- Characteristics:
- Marks the point where the temperature stops decreasing with altitude in the mesosphere and starts increasing in the thermosphere.
- Considered the coldest part of Earth’s atmosphere, with temperatures dropping to around -90°C (-130°F).
Energy Balance
Energy Balance
Energy balance in the atmosphere involves the transfer and transformation of energy through various processes. Understanding these processes is crucial for comprehending Earth’s climate system and the effects of phenomena such as global warming.
Conductive and Convective Heat Transfer
-
Conductive Heat Transfer:
- Definition: Transfer of heat through a material without the material itself moving.
- Mechanism: Heat flows from regions of higher temperature to regions of lower temperature due to molecular vibrations and collisions.
- Example: Heat conduction through the ground or building materials.
-
Convective Heat Transfer:
- Definition: Transfer of heat by the physical movement of a fluid (liquid or gas).
- Mechanism: Warm fluid rises and cool fluid sinks, creating a cycle that transfers heat.
- Types:
- Natural Convection: Caused by buoyancy differences due to temperature variations in the fluid.
- Forced Convection: Occurs when an external force (like a fan or pump) moves the fluid.
- Example: Warm air rising and cool air descending, forming convection currents in the atmosphere.
Radiation Heat Transfer
- Definition: Transfer of energy by electromagnetic waves (radiation).
- Mechanism: Energy is emitted by a source (such as the Sun) and absorbed by objects (such as Earth’s surface and atmosphere).
- Characteristics:
- Does not require a medium (can occur in a vacuum).
- Includes visible light, infrared radiation, and ultraviolet radiation.
- Example: Solar radiation heating the Earth’s surface.
Simple Global Temperature Model
-
Earth as a Black Body:
- Definition: A black body is an idealized object that absorbs all incident radiation and re-emits energy at a rate dependent on its temperature.
- Application: Assumes Earth absorbs all solar radiation and re-emits it as infrared radiation.
- Stefan-Boltzmann Law: Describes the power radiated from a black body in terms of its temperature: where is the Stefan-Boltzmann constant and is the absolute temperature.
-
Earth as Albedo:
- Definition: Albedo is the reflectivity of a surface, i.e., the fraction of incoming solar radiation that is reflected back into space.
- Application: Earth reflects some of the incoming solar radiation due to surfaces like ice, clouds, and deserts.
- Effect: Higher albedo means more reflection and less absorption of solar energy, which can influence global temperatures.
Problems
Greenhouse Effects
- Definition: Warming of Earth’s surface and lower atmosphere caused by the presence of greenhouse gases (GHGs) that trap heat.
- Mechanism:
- GHGs (like CO2, CH4, and water vapor) absorb infrared radiation emitted by Earth and re-emit it in all directions, including back towards Earth.
- This process reduces the amount of heat escaping into space, warming the planet.
- Consequences:
- Increased global temperatures.
- Melting of polar ice caps and glaciers.
- Rising sea levels.
- Changes in weather patterns and ecosystems.
Global Warming and Its Consequence
- Definition: The long-term rise in the average temperature of Earth’s climate system.
- Consequences:
- More frequent and severe weather events (hurricanes, droughts, heatwaves).
- Ocean acidification.
- Disruption of habitats and loss of biodiversity.
- Impact on agriculture, water supply, and human health.
Control of Global Warming
- Strategies:
- Reducing GHG emissions (using renewable energy, improving energy efficiency).
- Carbon capture and storage (CCS).
- Reforestation and afforestation.
- International agreements (Paris Agreement).
Earth’s Heat Budget
- Definition: The balance between incoming solar radiation and outgoing terrestrial radiation.
- Components:
- Incoming Solar Radiation: About 340 W/m² of solar energy reaches the top of the atmosphere.
- Reflected Solar Radiation: About 30% (albedo) is reflected back to space.
- Absorbed Solar Radiation: About 70% is absorbed by the atmosphere and surface.
- Outgoing Longwave Radiation: Earth emits infrared radiation to balance the absorbed solar energy.
- Balance: For a stable climate, the incoming and outgoing energy must be equal.
Lapse Rate
The lapse rate refers to the rate at which air temperature decreases with an increase in altitude. There are different types of lapse rates, each important for understanding weather patterns, atmospheric stability, and phenomena such as temperature inversions.
Ambient Lapse Rate
- Definition: The actual rate of temperature decrease with altitude in the atmosphere at a specific time and place.
- Characteristics:
- Can vary depending on weather conditions, geographic location, and time of day.
- Influenced by factors such as solar heating, radiative cooling, and atmospheric moisture.
- Measurement: Typically measured using weather balloons equipped with instruments called radiosondes.
Adiabatic Lapse Rate
- Definition: The rate of temperature change in a parcel of air as it moves up or down without exchanging heat with its surroundings (adiabatic process).
- Types:
- Dry Adiabatic Lapse Rate (DALR):
- Value: Approximately 9.8°C per kilometer (5.4°F per 1,000 feet).
- Application: Applies to unsaturated (dry) air parcels.
- Moist (Saturated) Adiabatic Lapse Rate (MALR):
- Value: Varies, but typically around 5°C to 6°C per kilometer (3°F to 3.3°F per 1,000 feet).
- Application: Applies to saturated air parcels (air at or near dew point, where condensation occurs).
- Reason for Variation: The release of latent heat during condensation warms the rising air parcel, reducing the lapse rate.
- Dry Adiabatic Lapse Rate (DALR):
Atmospheric Stability
- Definition: The tendency of the atmosphere to resist or enhance vertical motion of air parcels.
- Types:
- Stable Atmosphere: When the ambient lapse rate is less than the adiabatic lapse rate, suppressing vertical motion. This can lead to stratification and limited mixing.
- Unstable Atmosphere: When the ambient lapse rate is greater than the adiabatic lapse rate, promoting vertical motion. This can lead to convection, cloud formation, and potentially thunderstorms.
- Neutral Stability: When the ambient lapse rate is equal to the adiabatic lapse rate, resulting in neither suppression nor enhancement of vertical motion.
Temperature Inversion
- Definition: A reversal of the normal temperature decrease with altitude, where temperature increases with height over a certain layer of the atmosphere.
- Types:
- Radiation Inversion: =Forms near the ground on clear, calm nights when the ground cools rapidly by radiating heat, cooling the air directly above it=.
- Frontal Inversion: Occurs along weather fronts where warm air is forced over cooler air.
- Subsidence Inversion: Caused by sinking air that compresses and warms, often associated with high-pressure systems.
- Consequences:
- Traps pollutants and can lead to poor air quality and smog formation.
- Suppresses convection and cloud formation.
- Can create a stable layer that impacts aircraft flight and weather forecasting.
Atmospheric Dispersion
Atmospheric dispersion involves the spread of pollutants and other substances in the atmosphere.
Atmospheric Dispersion
Maximum Mixing Depth
- Definition: The maximum vertical distance in the atmosphere through which pollutants can be mixed.
- Importance: Determines how well pollutants disperse vertically; a greater mixing depth generally leads to better dispersion and lower concentrations at ground level.
- Factors Affecting Mixing Depth: Weather conditions, time of day, and geographical features.
Ventilation Coefficient
- Definition: A measure of the atmosphere’s ability to disperse pollutants, calculated as the product of the mixing height and average wind speed within the mixed layer.
- Formula: VC=MH×WS
- MH: Mixing Height (height to which pollutants are mixed)
- WS: Wind Speed
- Importance: High ventilation coefficient indicates better dispersion and dilution of pollutants
Effective Stack Height
- Definition: The actual height at which pollutants are effectively released into the atmosphere, accounting for both the physical stack height and the rise of the plume due to thermal buoyancy and momentum.
-
- Calculation:
- ** : Physical height of the stack.
- ΔH: Plume rise.
- Calculation:
- Importance: Higher effective stack height leads to better dispersion and reduced ground-level concentrations of pollutants.
Smokestack Plumes
- Types:
- Coning: Occurs in neutral stability conditions, where the plume spreads horizontally and vertically in a cone shape.
- Fanning: Occurs in stable conditions, where the plume spreads horizontally but not vertically, leading to limited dispersion.
- Looping: Occurs in unstable conditions, where the plume rises and falls in loops due to turbulent mixing.
- Lofting: Occurs when the atmosphere is stable below the plume but unstable above it, allowing the plume to rise and disperse above the inversion layer.
- Fumigation: Occurs when the atmosphere is unstable below the plume but stable above it, causing pollutants to be trapped near the ground.
Definition of Pollutants and Contaminants
Primary Pollutants
- Definition: Pollutants that are emitted directly from a source.
- Examples:
- Carbon monoxide (CO)
- Sulfur dioxide (SO2)
- Nitrogen oxides (NOx)
- Volatile organic compounds (VOCs)
- Particulate matter (PM10, PM2.5)
Secondary Pollutants
- Definition: Pollutants that form in the atmosphere through chemical reactions involving primary pollutants and other atmospheric components.
- Examples:
- Ozone (O3)
- Secondary particulate matter (e.g., sulfates, nitrates)
- Peroxyacyl nitrates (PANs)
Sources and Effects of Different Air Pollutants
Suspended Particulate Matter (PM)
- Sources: Combustion processes, industrial activities, vehicle emissions, natural sources (e.g., dust storms).
- Effects: Respiratory and cardiovascular diseases, reduced visibility, environmental damage.
Oxides of Carbon (CO, CO2)
- Sources: Incomplete combustion of fossil fuels, biomass burning.
- Effects: Carbon monoxide poisoning, contribution to greenhouse effect and global warming (CO2).
Oxides of Nitrogen (NO, NO2)
- Sources: Combustion processes, vehicle emissions, industrial activities.
- Effects: Respiratory problems, formation of ground-level ozone, acid rain.
Oxides of Sulfur (SO2)
- Sources: Fossil fuel combustion, industrial processes.
- Effects: Respiratory problems, acid rain, damage to vegetation and buildings.
Particulate PAN (Peroxyacyl Nitrates)
- Sources: Photochemical reactions involving VOCs and NOx.
- Effects: Eye irritation, respiratory issues, plant damage.
Smog
Photochemical Smog
- Definition: A type of air pollution produced by the reaction of sunlight with pollutants such as VOCs and NOx.
- Characteristics: Contains ozone, PANs, and other secondary pollutants.
- Effects: Respiratory problems, reduced visibility, damage to crops and materials.
London Smog
- Definition: A type of air pollution characterized by high levels of sulfur dioxide and particulate matter, typically from the burning of coal.
- Characteristics: Often occurs in cold weather with temperature inversions.
- Effects: Severe respiratory and cardiovascular health issues, historically linked to deadly smog events in industrial era London.
Ozone Layer Depletion
Ozone Layer
- Definition: The ozone layer is a region of the Earth's stratosphere that contains a high concentration of ozone (O3) molecules. It is located about 10 to 30 kilometers (6 to 19 miles) above the Earth's surface.
- Function: The ozone layer absorbs most of the Sun's harmful ultraviolet (UV) radiation, particularly UV-B and UV-C rays, protecting living organisms from DNA damage and other harmful effects.
Causes of Ozone Depletion
-
Chlorofluorocarbons (CFCs)
- Definition: CFCs are synthetic compounds containing chlorine, fluorine, and carbon. They were widely used in refrigeration, air conditioning, foam blowing, and aerosol propellants.
- Mechanism:
- CFCs are stable in the lower atmosphere (troposphere) but when they reach the stratosphere, they are broken down by UV radiation, releasing chlorine atoms.
- The chlorine atoms catalyze the destruction of ozone molecules through a series of chemical reactions.
- One chlorine atom can destroy thousands of ozone molecules before being deactivated.
-
Halons
- Definition: Halons are compounds containing bromine, fluorine, and carbon. They were used in fire extinguishers.
- Mechanism: Similar to CFCs, halons release bromine atoms in the stratosphere, which are even more efficient than chlorine in destroying ozone molecules.
-
Other Ozone-Depleting Substances (ODS)
- Examples: Carbon tetrachloride, methyl chloroform, and hydrochlorofluorocarbons (HCFCs).
- Mechanism: These substances also release chlorine or bromine atoms when broken down by UV radiation, contributing to ozone depletion.

Effects of Ozone Depletion
-
Increased UV Radiation
- Health Impacts:
- Increased risk of skin cancer (melanoma and non-melanoma).
- Higher incidence of cataracts and other eye damage.
- Suppressed immune system response.
- Environmental Impacts:
- Damage to phytoplankton, the foundation of the marine food web.
- Reduced crop yields and forest productivity.
- Negative effects on terrestrial and aquatic ecosystems.
- Health Impacts:
-
Climate Change
- Ozone depletion also affects atmospheric temperature and circulation patterns, contributing to climate change.
Control Measures and International Agreements (Read only if you have time)
-
Montreal Protocol
- Adoption: The Montreal Protocol on Substances that Deplete the Ozone Layer was adopted in 1987.
- Objective: To phase out the production and consumption of ozone-depleting substances (ODS).
- Success: The protocol is considered one of the most successful environmental agreements, with significant reductions in the use of CFCs and other ODS. The ozone layer is slowly recovering as a result.
-
Alternatives to ODS
- HCFCs and HFCs: Initially used as transitional substitutes for CFCs. HCFCs still have some ozone depletion potential, while HFCs do not deplete the ozone but are potent greenhouse gases.
- Natural Refrigerants: Ammonia, carbon dioxide, and hydrocarbons are being promoted as alternatives that do not deplete the ozone and have lower global warming potentials.
-
Continued Monitoring and Research
- Ongoing scientific research and monitoring are crucial to assess the health of the ozone layer and the effectiveness of regulatory measures.
- Satellite and ground-based observations are used to measure ozone concentrations and monitor recovery trends.
Standards and Control Measures
Industrial, Commercial, and Residential Air Quality Standards
Standards
- Definition: Air quality standards are regulatory limits set on the concentration of pollutants in the air to protect human health and the environment.
- Purpose: These standards are designed to reduce the health risks associated with air pollution, such as respiratory and cardiovascular diseases, and to protect the environment from damage due to pollutants.
Types of Standards
-
National Ambient Air Quality Standards (NAAQS)
- Definition: Set by governments (e.g., the U.S. Environmental Protection Agency - EPA) to regulate pollutants considered harmful to public health and the environment.
- Primary Standards: Protect human health, including sensitive populations such as children, the elderly, and individuals with respiratory conditions.
- Secondary Standards: Protect public welfare, including protection against decreased visibility, damage to animals, crops, vegetation, and buildings.
-
World Health Organization (WHO) Guidelines
- Definition: Provide global guidelines on air quality to assist countries in establishing their air quality standards.
- Purpose: To promote health and protect populations by reducing the burden of disease caused by air pollution.
Control Measures
Technologies and Practices
-
Electrostatic Precipitator (ESP)
- Function: Removes fine particulate matter (PM) from exhaust gases.
- Mechanism: Uses electrical charges to attract and capture particles.
- Applications: Commonly used in power plants and industrial facilities.
-
Cyclone Separator
- Function: Removes large particulate matter from gas streams.
- Mechanism: Utilizes centrifugal force to separate particles from the gas stream.
- Applications: Used in industries like cement manufacturing and metal processing.
-
Bag House (Fabric Filter)
- Function: Captures particulate matter from exhaust gases by filtering them through fabric bags.
- Mechanism: Particles are trapped on the fabric surface as gases pass through.
- Applications: Widely used in industries such as pharmaceuticals, chemicals, and food processing.
-
Catalytic Converter
- Function: Reduces emissions of harmful gases from vehicle exhaust.
- Mechanism: Uses a catalyst to convert harmful gases (e.g., CO, NOx, hydrocarbons) into less harmful substances (e.g., CO2, H2O, N2).
- Applications: Standard in all modern vehicles.
-
Scrubber (Venturi Scrubber)
- Function: Removes pollutants from exhaust gases.
- Mechanism: Uses a liquid (often water) to wash pollutants out of the gas stream.
- Applications: Effective for removing both particulate matter and gaseous pollutants; used in chemical plants, refineries, and manufacturing industries.
Control Measures in Practice (Read only if you have time/ common sense stuff)
-
Industrial Emission Standards
- Regulations: Industries are required to adhere to emission standards that limit the release of pollutants.
- Monitoring and Reporting: Regular monitoring and reporting of emissions are mandated to ensure compliance.
-
Vehicle Emission Standards
- Regulations: Standards are set to control the amount of pollutants that vehicles can emit.
- Technological Requirements: Adoption of technologies like catalytic converters, fuel injection systems, and electric or hybrid engines.
-
Energy Efficiency and Renewable Energy
- Purpose: Reducing reliance on fossil fuels and lowering emissions.
- Technologies: Solar panels, wind turbines, energy-efficient appliances and industrial processes.
-
Urban Planning and Green Spaces
- Objective: Reducing air pollution in urban areas by improving infrastructure and increasing vegetation.
- Measures: Creation of parks, green belts, and promoting public transportation and non-motorized transport.
Benefits of Standards and Control Measures
- Health Protection: Reducing exposure to harmful pollutants decreases the incidence of respiratory and cardiovascular diseases, cancer, and other health conditions.
- Environmental Protection: Limiting pollutant emissions helps to protect ecosystems, improve visibility, and reduce acid rain and eutrophication.
- Economic Benefits: Healthier populations lead to reduced healthcare costs and increased productivity. Improved environmental quality can enhance tourism and property values.