Biomolecules: Overview
Biomolecules are organic molecules that are crucial to the structure and function of living organisms. They include a wide variety of molecules such as carbohydrates, proteins, nucleic acids, and lipids. Despite the vast diversity of life, these molecules are composed of a limited number of types of monomeric units that assemble into complex polymeric structures.
Monomeric Units
Monomeric units are small, basic molecules that can join together to form larger, complex structures called polymers. Here are some key types of monomeric units:
-
Monosaccharides (Simple Sugars)
- Examples: Glucose, fructose, galactose.
- Structure: Typically have the formula (CH₂O)n, where n is usually 3-7.
- Function: Serve as an immediate source of energy for cells and as building blocks for more complex carbohydrates.
-
Amino Acids
- Examples: Glycine, alanine, valine.
- Structure: Each amino acid has a central carbon (alpha carbon) bonded to an amino group (-NH₂), a carboxyl group (-COOH), a hydrogen atom, and a variable R group (side chain).
- Function: Building blocks of proteins, involved in nearly all cellular processes.
-
Nucleotides
- Examples: Adenine, thymine, cytosine, guanine, uracil.
- Structure: Composed of three parts: a nitrogenous base, a five-carbon sugar (ribose or deoxyribose), and one or more phosphate groups.
- Function: Form the genetic material of cells (DNA and RNA), and play roles in energy transfer (ATP).
-
Fatty Acids
- Examples: Palmitic acid, oleic acid, linoleic acid.
- Structure: Consist of a long hydrocarbon chain with a terminal carboxyl group (-COOH).
- Function: Building blocks of lipids, essential for energy storage, membrane structure, and signaling.
Polymeric Units
Polymers are large, complex molecules made up of repeating monomeric units linked together by covalent bonds. Here are some key types of polymeric units:
-
Polysaccharides
- Examples: Starch, glycogen, cellulose.
- Structure: Long chains of monosaccharides linked by glycosidic bonds.
- Function:
- Starch: Energy storage in plants.
- Glycogen: Energy storage in animals.
- Cellulose: Structural component in plant cell walls.
-
Proteins
- Examples: Enzymes, antibodies, hemoglobin.
- Structure: Polymers of amino acids linked by peptide bonds. Proteins fold into specific three-dimensional shapes critical for their function.
- Function: Catalyze biochemical reactions, provide structural support, transport molecules, regulate processes, and more.
-
Nucleic Acids
- Examples: DNA, RNA.
- Structure: Polymers of nucleotides linked by phosphodiester bonds.
- Function:
- DNA: Stores genetic information.
- RNA: Transfers genetic information from DNA to the protein synthesis machinery and plays roles in regulation and catalysis.
-
Lipids
- Examples: Triglycerides, phospholipids, steroids.
- Structure: Often formed from fatty acids and glycerol, or other components like phosphate groups in phospholipids.
- Function: Energy storage (triglycerides), make up cell membranes (phospholipids), and serve as signaling molecules (steroids).
Sugar, Starch and Cellulose
Sugar
Sugars are the simplest form of carbohydrates and are crucial as an energy source for living organisms. They can be classified into two main types: monosaccharides and disaccharides.
-
Monosaccharides (Simple Sugars)
- Examples: Glucose, fructose, galactose.
- Structure: Consist of a single sugar unit with the general formula (CH₂O)n, where n is usually 3-7. For instance, glucose has the formula C₆H₁₂O₆.
- Function: Serve as the primary energy source for cells. Glucose, for example, is a critical energy source that is metabolized during cellular respiration to produce ATP.
-
Disaccharides
- Examples: Sucrose, lactose, maltose.
- Structure: Composed of two monosaccharide units linked by a glycosidic bond. For example, sucrose (table sugar) is made up of glucose and fructose.
- Function: Provide a source of energy and also serve as transportable forms of sugars in plants (e.g., sucrose).
Starch
Starch is a polysaccharide that serves as a major storage form of energy in plants.
-
Structure:
- Starch is composed of two types of glucose polymers: amylose and amylopectin.
- Amylose: Consists of long, unbranched chains of glucose molecules connected by α(1→4) glycosidic bonds.
- Amylopectin: Contains branched chains of glucose molecules, with α(1→4) glycosidic bonds along the chains and α(1→6) glycosidic bonds at the branch points.
-
Function:
- Acts as an energy reserve that plants can break down into glucose molecules when needed.
- In the human diet, starch is an important source of carbohydrates, broken down into simpler sugars by digestive enzymes.
Cellulose
Cellulose is another polysaccharide but with a structural role rather than a storage role.
-
Structure:
- Composed of long, unbranched chains of glucose molecules connected by β(1→4) glycosidic bonds.
- The β(1→4) linkage results in a straight, rigid structure that allows cellulose molecules to pack tightly together, forming strong fibers.
-
Function:
- Provides structural support in plant cell walls, giving rigidity and strength to plants.
- In nature, cellulose is the most abundant organic polymer.
- Unlike starch, cellulose cannot be digested by humans because we lack the enzyme (cellulase) to break the β(1→4) glycosidic bonds. However, some herbivores and microorganisms have this enzyme and can digest cellulose.
Comparison and Importance
-
Chemical Composition: All three (sugars, starch, and cellulose) are made up of glucose units, but the type of glycosidic bond and the structure they form differ.
- Sugars: Simple structures with quick energy release.
- Starch: Energy storage with both unbranched (amylose) and branched (amylopectin) structures, allowing for efficient energy release.
- Cellulose: Structural component with high tensile strength due to β(1→4) linkages.
-
Biological Roles:
- Sugars: Immediate energy source and building blocks for other molecules.
- Starch: Long-term energy storage in plants, major dietary carbohydrate for humans.
- Cellulose: Structural support in plants, dietary fiber for humans.
Digestion and Utilization
- Sugars: Easily digested and absorbed by the body. Monosaccharides are directly absorbed into the bloodstream, while disaccharides are broken down into monosaccharides by specific enzymes before absorption.
- Starch: Broken down by amylase enzymes in saliva and the small intestine into maltose and then into glucose, which is absorbed into the bloodstream.
- Cellulose: Indigestible by humans, but it aids in digestion as dietary fiber, promoting healthy bowel movements and preventing constipation.
Amino acids and Proteins
Amino acids
Amino acids are the building blocks of proteins. There are 20 standard amino acids that are encoded by the genetic code.
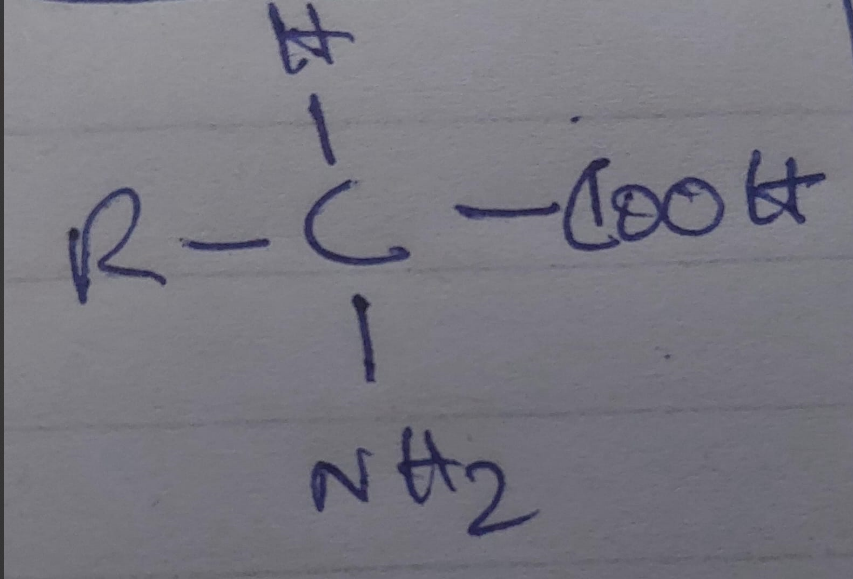
General structure of an amino acid
-
Structure of Amino Acids:
- Central (Alpha) Carbon (Cα): The central atom to which all other components are attached.
- Amino Group (-NH₂): A basic group that can accept a proton.
- Carboxyl Group (-COOH): An acidic group that can donate a proton.
- Hydrogen Atom (H): Attached to the central carbon.
- R Group (Side Chain): The variable group that defines the specific properties of each amino acid. The R group can vary in size, shape, charge, hydrophobicity, and reactivity.
-
Classification of Amino Acids:
- Nonpolar (Hydrophobic): e.g., Valine, Leucine, Isoleucine.
- Polar (Hydrophilic): e.g., Serine, Threonine, Asparagine.
- Charged (Acidic and Basic): e.g., Aspartic acid (acidic), Lysine (basic).
- Special Function: e.g., Cysteine (forms disulfide bonds), Glycine (smallest, fits in tight spaces).
-
Essential vs. Non-essential Amino Acids:
- Essential: Cannot be synthesized by the body and must be obtained from the diet (e.g., Leucine, Isoleucine, Valine).
- Non-essential: Can be synthesized by the body (e.g., Alanine, Asparagine).
Proteins
Proteins are large, complex molecules composed of one or more chains of amino acids (polypeptides). They perform a vast array of functions within organisms.
-
Structure of Proteins(Skip if too hard):
- Primary Structure: The linear sequence of amino acids in a polypeptide chain, held together by peptide bonds.
- Secondary Structure: Localized folding into structures such as alpha helices and beta sheets, stabilized by hydrogen bonds.
- Tertiary Structure: The overall three-dimensional shape of a single polypeptide chain, stabilized by various interactions, including hydrogen bonds, ionic bonds, hydrophobic interactions, and disulfide bonds.
- Quaternary Structure: The assembly of multiple polypeptide chains (subunits) into a functional protein complex. Not all proteins have quaternary structure.
-
Types of Proteins:
- Enzymes: Catalyze biochemical reactions (e.g., DNA polymerase).
- Structural Proteins: Provide support and shape to cells and organisms (e.g., collagen, keratin).
- Transport Proteins: Carry molecules across membranes or through the body (e.g., hemoglobin, which transports oxygen).
- Motor Proteins: Involved in movement (e.g., myosin in muscles).
- Signaling Proteins: Involved in cell communication (e.g., insulin, a hormone).
- Defensive Proteins: Protect the organism (e.g., antibodies).
-
Functions of Proteins:
- Catalysis: Enzymes speed up chemical reactions without being consumed.
- Structure: Provide structural integrity and support to cells and tissues.
- Transport: Bind and carry small molecules and ions across cell membranes and throughout the body.
- Signaling: Hormones and receptors transmit signals to regulate biological processes.
- Movement: Actin and myosin are involved in muscle contraction.
- Defense: Antibodies recognize and neutralize foreign invaders like bacteria and viruses.
- Regulation: Regulatory proteins control gene expression and cellular processes.
Nucleotides, DNA and RNA
Nucleotides
Nucleotides are the building blocks of nucleic acids (DNA and RNA). Each nucleotide consists of three components:
-
Nitrogenous Base:
- Purines: Adenine (A) and Guanine (G) - larger, double-ring structures.
- Pyrimidines: Cytosine (C), Thymine (T) in DNA, and Uracil (U) in RNA - smaller, single-ring structures.
-
Five-Carbon Sugar (Pentose):
- Deoxyribose: Found in DNA (lacks an oxygen atom at the 2’ position).
- Ribose: Found in RNA (has an OH group at the 2’ position).
-
Phosphate Group:
- One or more phosphate groups are attached to the 5’ carbon of the sugar.
DNA (Deoxyribonucleic Acid)
DNA is the molecule that stores genetic information in all living organisms and many viruses.
-
Structure:
- Double Helix: Two strands of nucleotides coiled around each other, forming a right-handed helix.
- Backbone: Alternating sugar and phosphate groups connected by phosphodiester bonds.
- Base Pairing: Nitrogenous bases on opposite strands pair via hydrogen bonds:
- Adenine (A) pairs with Thymine (T) via two hydrogen bonds.
- Guanine (G) pairs with Cytosine (C) via three hydrogen bonds.
- Antiparallel Strands: The two strands run in opposite directions (5’ to 3’ and 3’ to 5’).
-
==Functions==:
- Genetic Information Storage: Encodes the instructions for building and maintaining an organism.
- Replication: Allows genetic information to be copied and passed on to daughter cells during cell division.
- Transcription: Template for the synthesis of RNA, which in turn is used to produce proteins.
RNA (Ribonucleic Acid)
RNA plays several roles in the expression of genetic information.
-
Structure:
- Single-Stranded: RNA is typically single-stranded but can form secondary structures (e.g., hairpins, loops).
- Backbone: Alternating ribose sugar and phosphate groups.
- Base Pairing: In RNA, Adenine (A) pairs with Uracil (U) instead of Thymine (T). Guanine (G) still pairs with Cytosine (C).
-
==Types and Functions==:
- mRNA (Messenger RNA): Carries the genetic code from DNA to the ribosomes for protein synthesis.
- tRNA (Transfer RNA): Brings amino acids to the ribosome during protein synthesis and matches them to the codons in mRNA.
- rRNA (Ribosomal RNA): Structural and catalytic component of ribosomes, which are the sites of protein synthesis.
- Other RNA Molecules: Includes snRNA (small nuclear RNA), miRNA (microRNA), siRNA (small interfering RNA), and others involved in various regulatory and catalytic functions.
Two-Carbon Units
Two-carbon units, often referred to in the context of metabolism, are fundamental components in various biochemical pathways. The most notable two-carbon unit is the acetyl group.
-
Acetyl Group:
- Structure: The acetyl group has the chemical formula CH₃CO-.
- Formation: Often formed from the breakdown of carbohydrates, fats, and proteins.
- Function: Acetyl groups play a key role in the citric acid cycle (Krebs cycle), which is crucial for cellular respiration and energy production.
Lipids
Lipids are a diverse group of hydrophobic or amphiphilic molecules, which include fats, oils, waxes, phospholipids, and steroids. They play essential roles in energy storage, cell membrane structure, and signaling.
-
Triglycerides (Fats and Oils):
- Structure: Composed of one glycerol molecule bonded to three fatty acids via ester bonds.
- Function:
- Energy Storage: Provide a dense form of energy storage, yielding more than twice the energy per gram compared to carbohydrates and proteins.
- Insulation and Protection: In animals, triglycerides serve as insulation against cold temperatures and provide cushioning to protect vital organs.
-
Phospholipids:
- Structure: Consist of a glycerol backbone, two fatty acid tails (hydrophobic), and a phosphate group attached to a polar head group (hydrophilic).
- Function:
- Cell Membranes: Form the bilayer structure of cell membranes, creating a barrier that separates the interior of the cell from the external environment. The hydrophobic tails face inward, shielding them from water, while the hydrophilic heads face outward.
- Membrane Fluidity: Contribute to the fluidity and flexibility of cell membranes.
-
Steroids:
- Structure: Composed of four fused carbon rings with various functional groups attached.
- Function:
- Hormones: Steroids such as cholesterol, testosterone, and estrogen play key roles in signaling and regulation of physiological processes.
- Membrane Component: Cholesterol is a crucial component of cell membranes, contributing to membrane fluidity and stability.
-
Fatty Acids:
- Structure: Long hydrocarbon chains with a terminal carboxyl group. They can be saturated (no double bonds) or unsaturated (one or more double bonds).
- Function:
- Energy Source: Fatty acids are oxidized to produce ATP.
- Building Blocks: Serve as building blocks for more complex lipids, including phospholipids and triglycerides.